
PDF) Clinical study reports of randomised controlled trials: An exploratory review of previously confidential industry reports
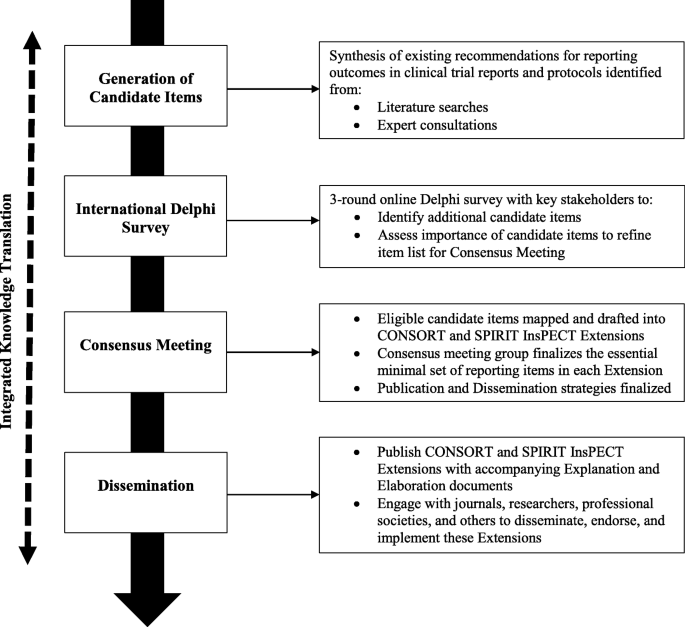
Improving outcome reporting in clinical trial reports and protocols: study protocol for the Instrument for reporting Planned Endpoints in Clinical Trials (InsPECT) | Trials | Full Text
CORE Reference: New guidelines of preparing clinical study reports for Japanese biopharmaceu=cal companies seeking interna=

Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI Extension | The BMJ
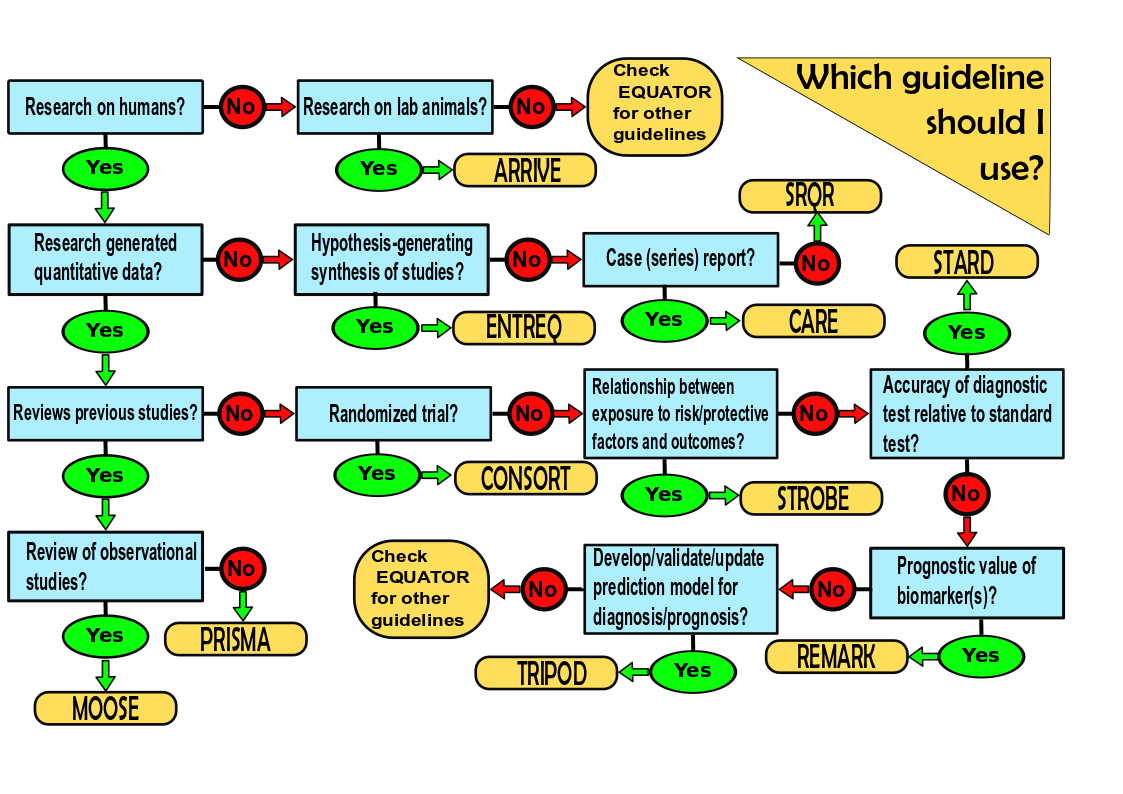
Biomedical study reporting guidelines - Essential guides to writing high-quality scientific reports - SciMeditor
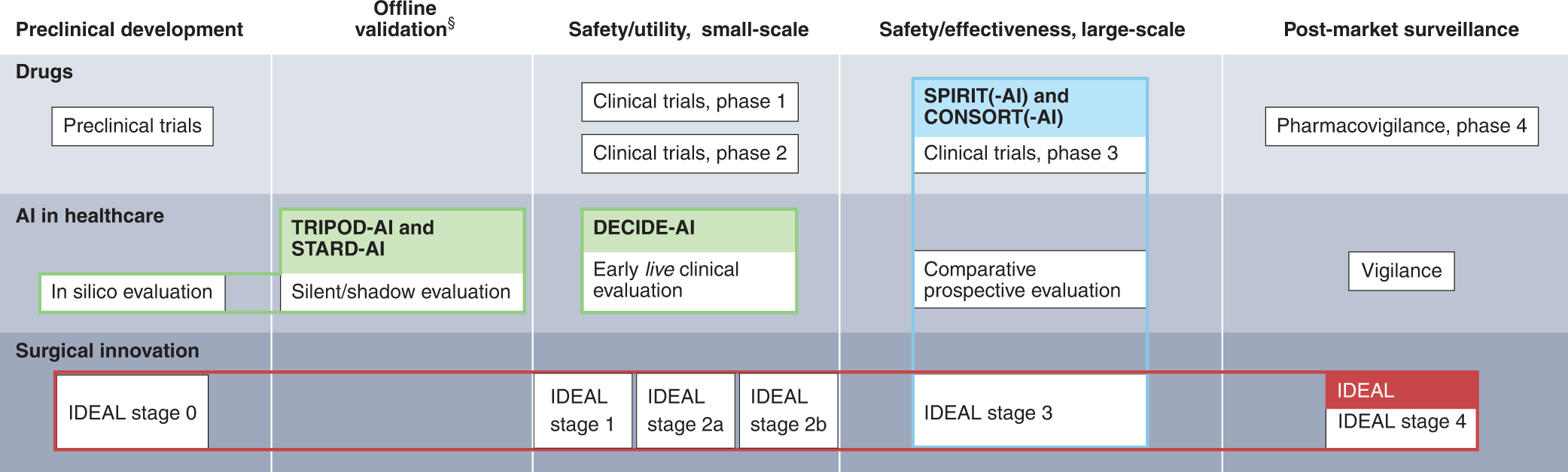
Reporting guideline for the early-stage clinical evaluation of decision support systems driven by artificial intelligence: DECIDE-AI | Nature Medicine

Construction of study sample comprising the 50 most recent clinical... | Download Scientific Diagram
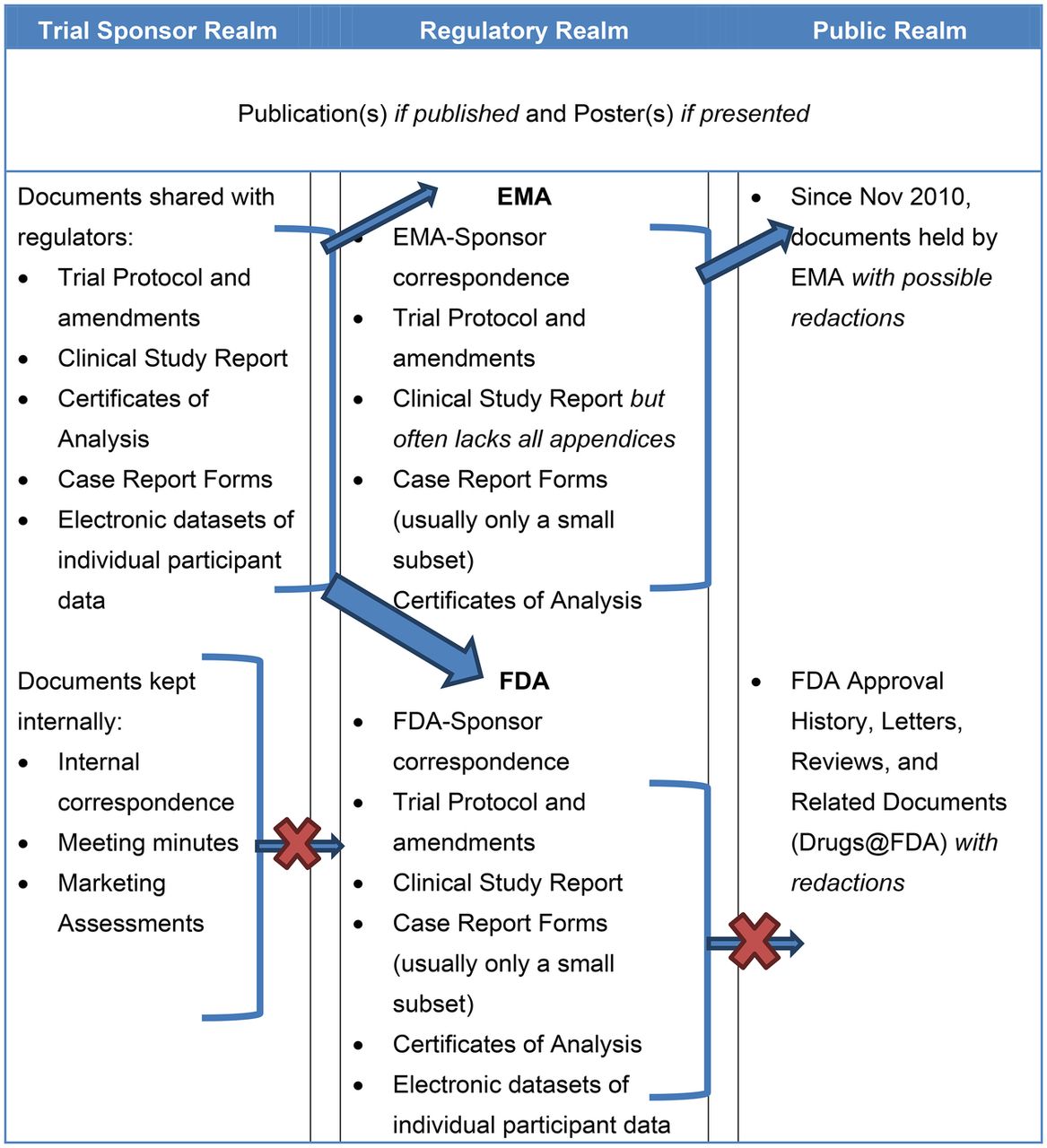

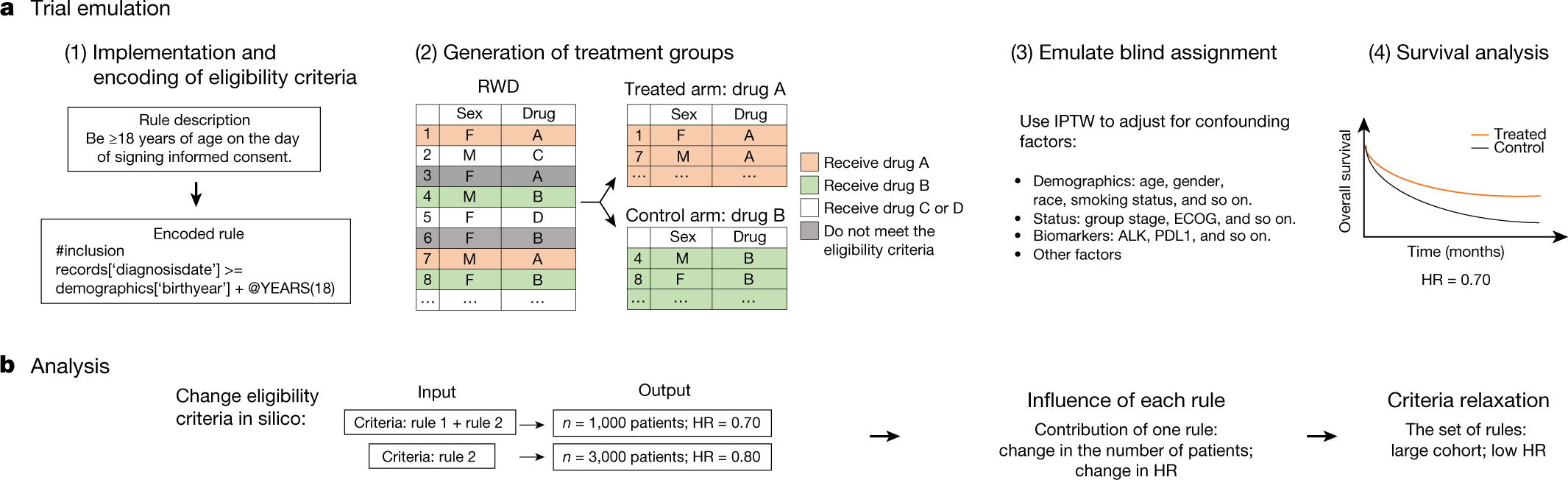

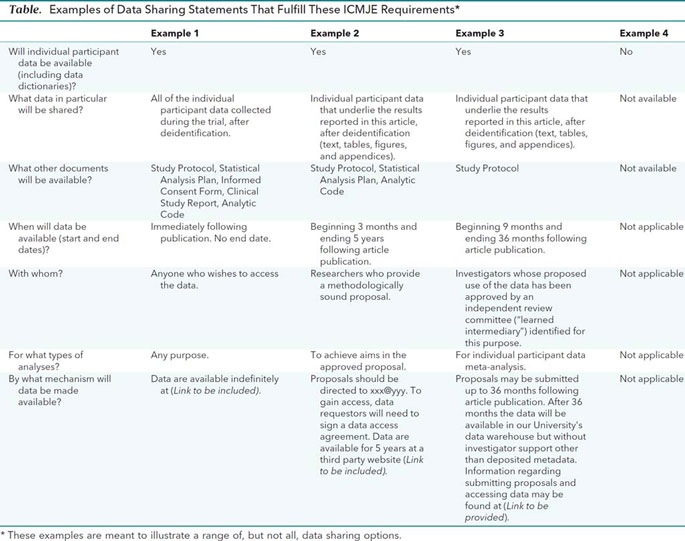












![PDF] Effective authoring of clinical study reports: A companion guide | Semantic Scholar PDF] Effective authoring of clinical study reports: A companion guide | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/2f8d38c9afbd7fce7a7635cf459a7989c82a753a/5-Table1-1.png)