Energies | Free Full-Text | Separation of the Mixture 2-Propanol + Water by Heterogeneous Azeotropic Distillation with Isooctane as an Entrainer | HTML
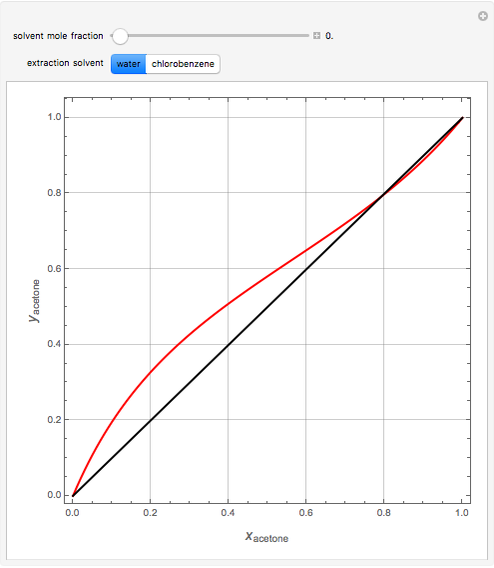
Breaking the Acetone-Methanol Azeotrope with Different Extraction Solvents - Wolfram Demonstrations Project
Is a 50/50 mixture of acetone and water an azeotrope? Also, why does the first drop of destillate form at 40C? - Quora
Is a 50/50 mixture of acetone and water an azeotrope? Also, why does the first drop of destillate form at 40C? - Quora

SciELO - Brasil - THERMODYNAMIC TOPOLOGICAL ANALYSIS OF EXTRACTIVE DISTILLATION OF MAXIMUM BOILING AZEOTROPES THERMODYNAMIC TOPOLOGICAL ANALYSIS OF EXTRACTIVE DISTILLATION OF MAXIMUM BOILING AZEOTROPES

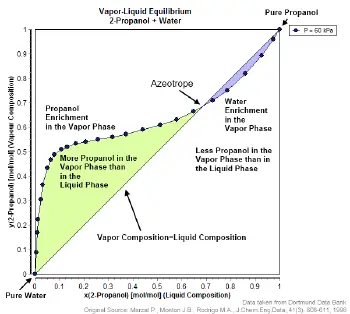




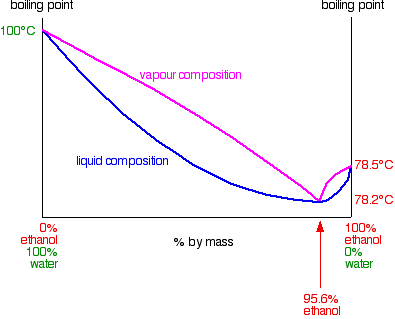
![PDF] Separation of Water-Acetone Mixture Using Suitable Entrainer (Simulation) | Semantic Scholar PDF] Separation of Water-Acetone Mixture Using Suitable Entrainer (Simulation) | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/3b24cb3fdaba29b0182f3943cf35a512430d1196/29-Table3-2-1.png)


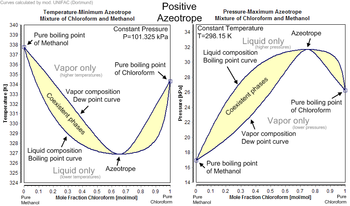
![PDF] Separation of Water-Acetone Mixture Using Suitable Entrainer (Simulation) | Semantic Scholar PDF] Separation of Water-Acetone Mixture Using Suitable Entrainer (Simulation) | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/3b24cb3fdaba29b0182f3943cf35a512430d1196/38-Table4-2-1.png)